Intermediates + Impurities
- Cetirizine Hydrochloride
- Doxazosin Mesylate
- Pazufloxacin Mesylate
- Omadacycline Tosylate
- Perampanel Intermediates
- Brexpiprazole
- Selexipag
- Lumateperone Tosylate
- Gadoxetate Disodium
- Suzetrigine VX-548
- Fezolinetant
- Daridorexant Hydrochloride
- Lemborexant
- Brinzolamide
- Brensocatib AZD-7986
- Seladelpar lysine dihydrate MBX-8052
- Lifitegrast
- Diquafosol Tetrasodium
- Lefamulin Acetate
- Netupitant
- Brivaracetam
- Bedaquiline Fumurate
- Lomefloxacin Hydrochloride
- Palonosetron Hydrochloride
- Suvorexant Intermediates
- Tedizolid Phosphate
- Vonoprazan Fumurate
- Valbenazine Tosylate
- Ozanimod Hydrochloride
- Netasudil Dimesylate
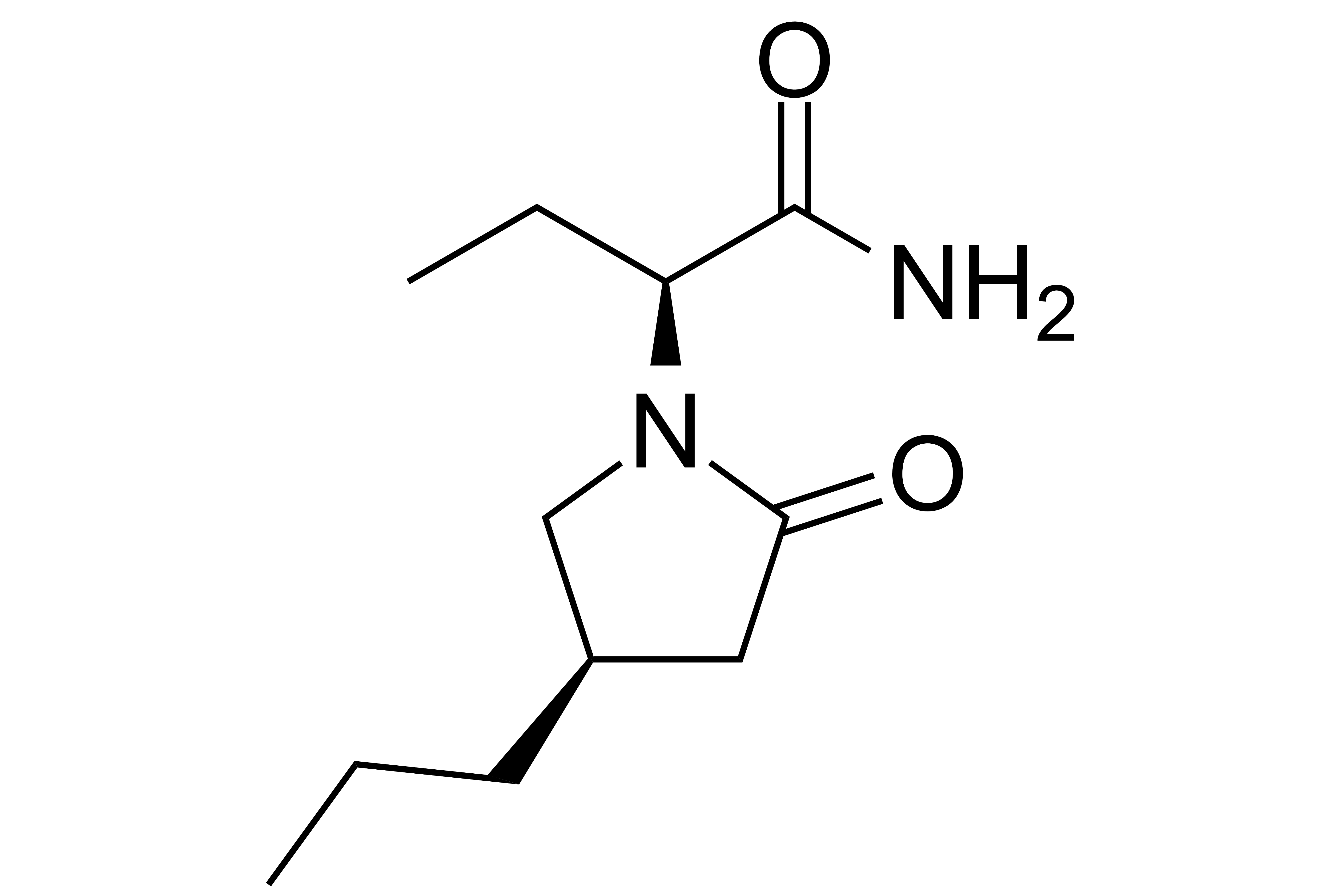
Brivaracetam
-
 Details >>
Details >>Brivaracetam
【Chemical Name】Brivaracetam
【Original】UCB
【Time to market】2016.02.18
【Patents expire】2021.02.21
【Dosage and Usage】Tablets:10 mg, 25 mg, 50 mg, 75 mg, and 100 mg,Oral solution:10 mg/mL,
Injection: 50mg/5 mL single-dose vial,used for the treatment of partial- onset seizures in patients 1 month of age and older


