Netasudil Dimesylate
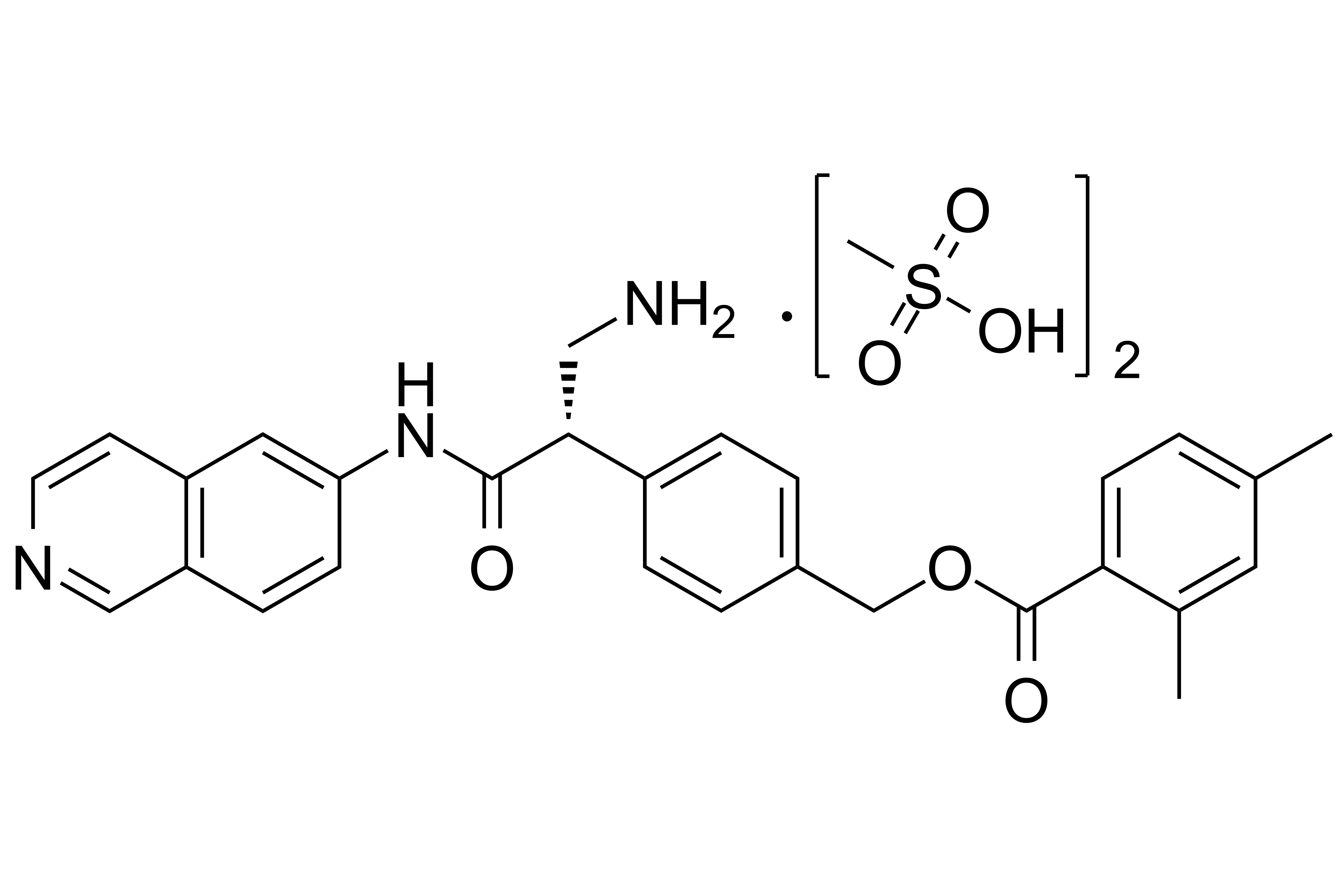
Netarsudil Dimesylate
【Chemical Name】Netarsudil Dimesylate
【Original】Aerie Pharmaceuticals
【Time to market 】2017.12.18
【Patents expire】2026年7月11日。
【Use】0.02% netarsudil eye drops for the treatment of open-angle glaucoma or high intraocular pressure.
Netarsudil Dimesylate
一、Product Overview
Netarsudil Dimesylate, developed by Aerie Pharmaceuticals as a drug for glaucoma or high intraocular pressure, was approved by the us food and drug administration (FDA) on December 18, 2017. The compound's patent expires in 2026.
二、Main products
Description | Structural Formula | CAS No. | Category |
 | 1422144-42-0 | API | |
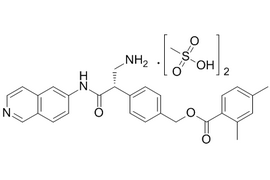
(S)-3-((tert-butoxycarbonyl)amino)-2-(4-(((2,4-dimethylbenzoyl)oxy)methyl)phenyl)propanoic acid | 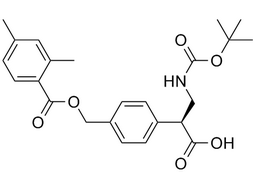 | 2097334-20-6 | Intermediate |
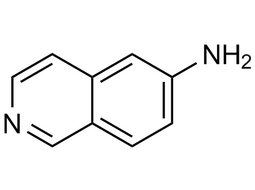 | 23687-26-5 | Intermediate | |
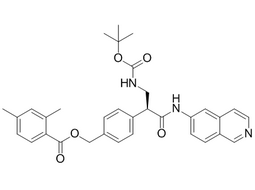 | 1253955-19-9 | Intermediate |
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.


