Valbenazine Tosylate
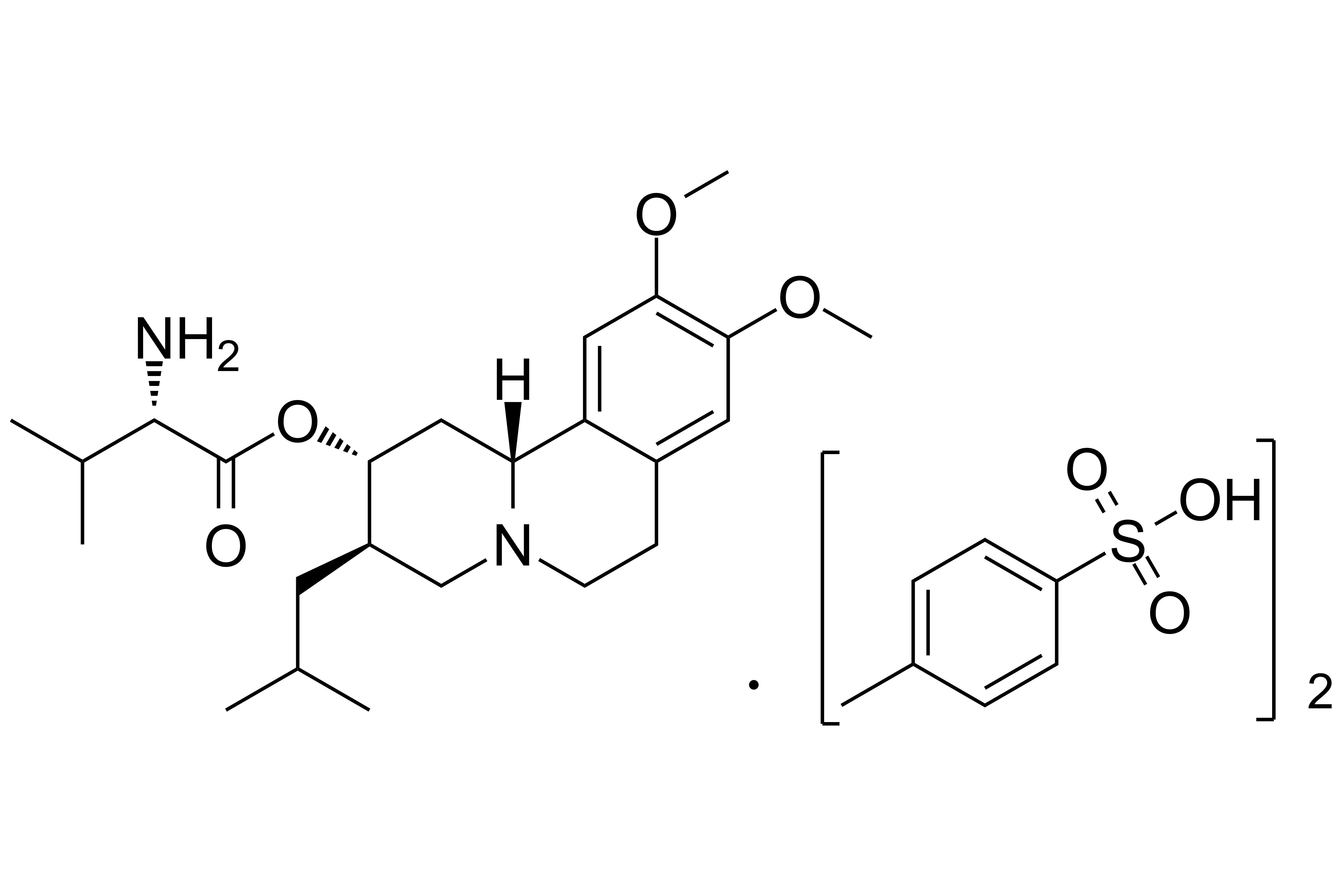
Valbenazine Tosylate
【Chemical Name】Valbenazine Tosylate
【Original】Neurocrine Biosciences
【Time to market】2017.04.11
【Patents expire】2029.10.06
【Dosage and Usage】Capsules: 40 mg, 60 mg and 80 mg. Used for the treatment of
adult - tardive dyskinesia and chorea associated with Huntington’s disease.
Valbenazine Tosylate
一、Product Overview
Valbenazine, developed by Neurocrine Biosciences for the treatment of adult - tardive dyskinesia and chorea associated with Huntington’s disease., was approved by the Food and Drug Administration (FDA) on April 11, 2017 and its patent expires in 2029.
二、Main Product
Description | Structural Formula | CAS No. | Category |
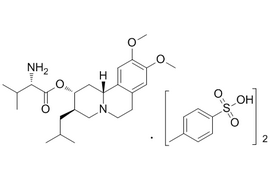 | 1639208-54-0 | API | |
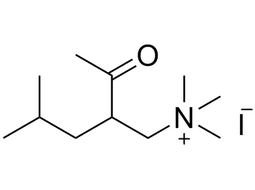 | 1069-62-1 | Intermediate | |
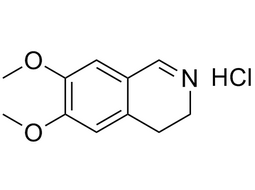 | 20232-39-7 | Intermediate | |
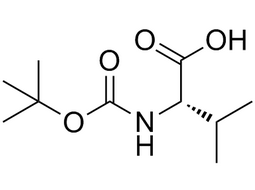 | 13734-41-3 | Intermediate |
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.


