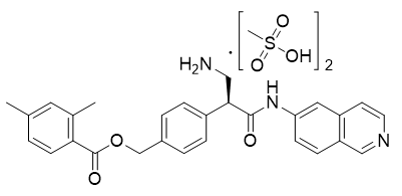
Netasudil Dimesylate
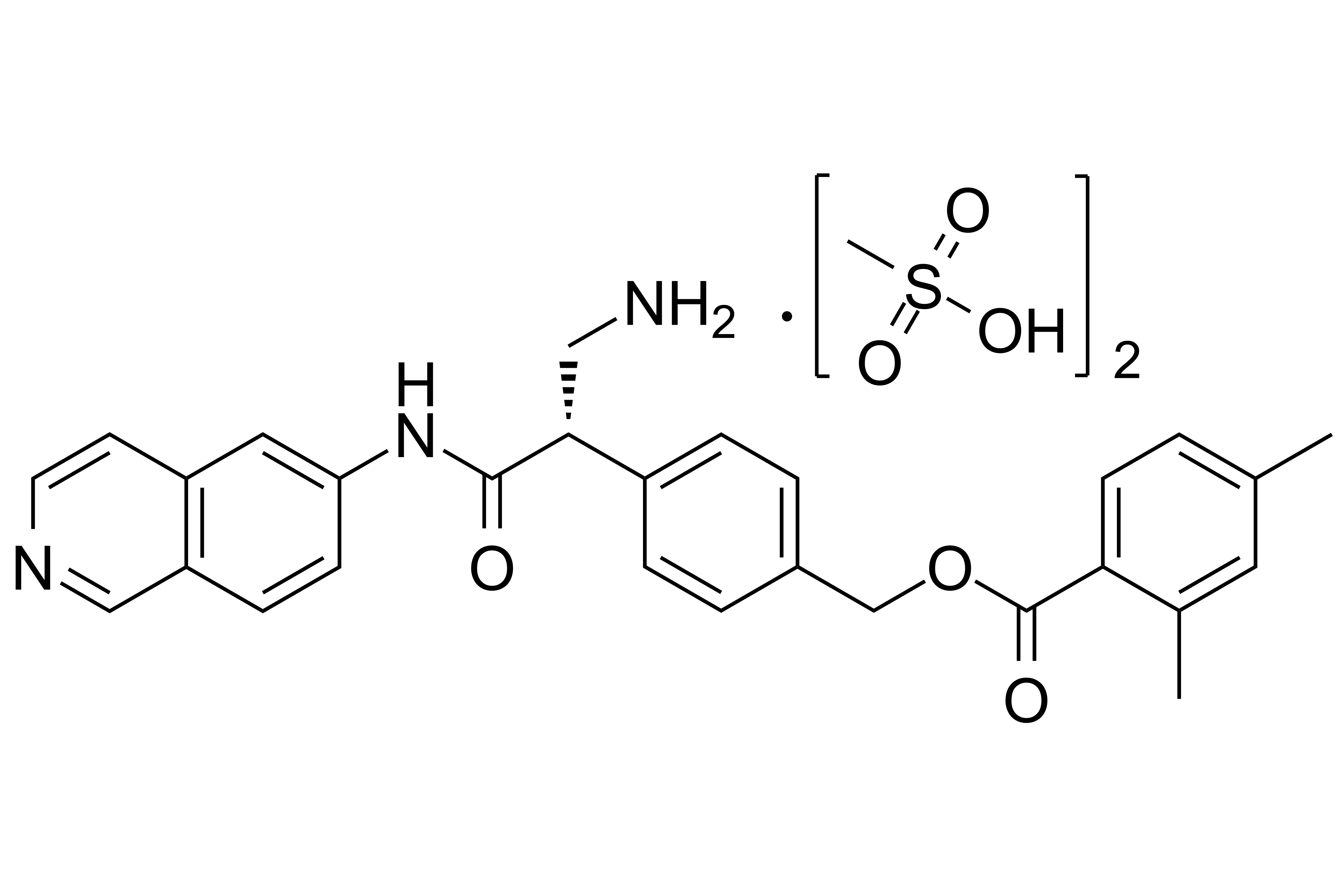
Netarsudil Dimesylate
【Chemical Name】Netarsudil Dimesylate
【Original】Aerie Pharmaceuticals
【Time to market 】2017.12.18
【Patents expire】2026.07.11
【Dosage and Usage】0.02% netarsudil eye drops for the treatment of open-angle glaucoma or ocular hypertension.
Netarsudil Dimesylate
一、Product Overview
Netarsudil Dimesylate, developed by Aerie Pharmaceuticals as a drug for glaucoma or high intraocular pressure, was approved by the us food and drug administration (FDA) on December 18, 2017. The compound's patent expires in 2026.
二、Main products
Structural Formula | CAS No. | Category | |
| 1422144-42-0 | API | |
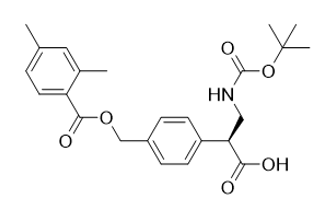
(S)-3-((tert-butoxycarbonyl)amino)-2-(4-(((2,4-dimethylbenzoyl)oxy)methyl)phenyl)propanoic acid |
| 2097334-20-6 | Intermediate |
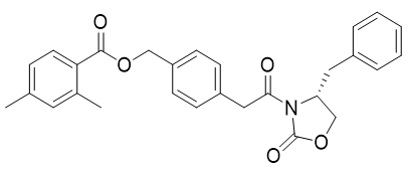
| 23687-26-5 | Intermediate | |
(R)-4-(2-(4-benzyl-2-oxooxazolidin-3-yl)-2-oxoethyl)benzyl 2,4-dimethylbenzoate |
| 2097334-18-2 | Intermediate |
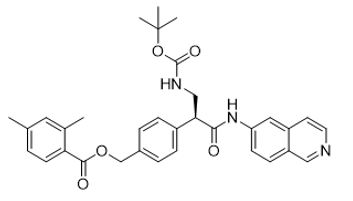 | 1253955-19-9 | Intermediate |
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.