Tofacitinib Citrate

Tofacitinib Citrate
【Chemical Name】Tofacitinib Citrate
【Original】Pfizer
【Time to market】2012.11.6
【Patents expire】2020.12.8
【Use】Oral administration of 5mg tablets for rheumatoid arthritis.
Tofacitinib citrate
一、Product Overview
Product background: Tofacitinib citrate for the occasional method developed by Pfizer (Pfizer), used in the treatment of adult patients with methotrexate response inadequate or intolerance of moderate to severe active rheumatoid arthritis, on November 6, 2012 by the us food and drug administration (FDA) approved, after in March 25, 2013, the Japanese pharmaceuticals (PMDA) approved medical equipment integrated mechanism, compound patent expires in November 2020.
二、Main products
Description | Structural Formula | CAS No. | Category |
Tofacitinib Citrate | 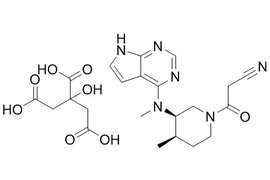 | 540737-29-9 | API |
(3R,4R)- Cis-1-benzyl-4-methyl-3-(methylamino)piperidine dihydrochloride | 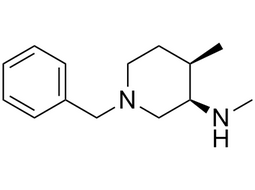 | 1062580-52-2 | intermediates |
4-chloro-7H-pyrrolo[2,3-d]pyrimidine | 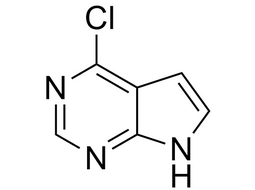 | 3680-69-1 | intermediates |
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.


