Selexipag
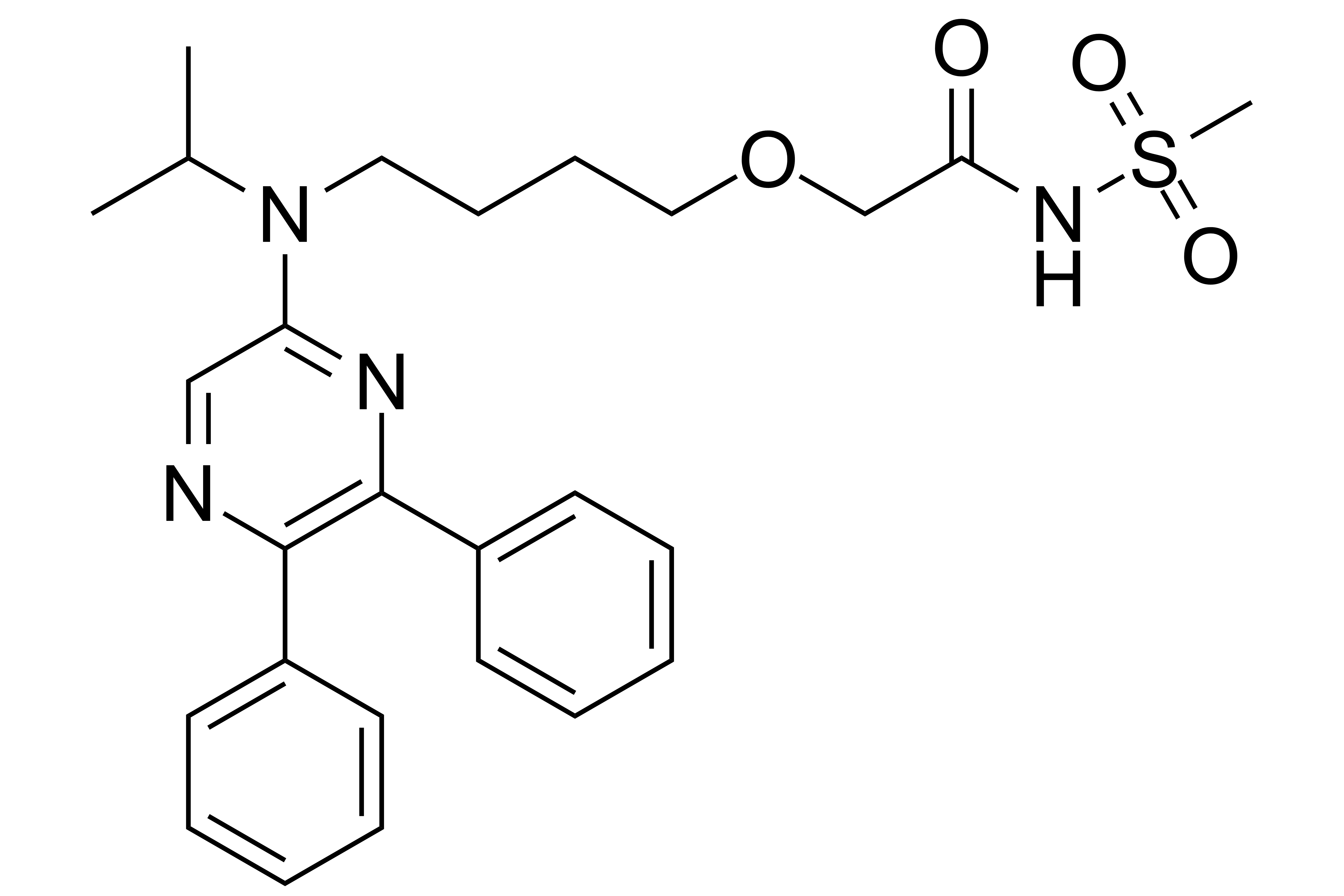
Selexipag
【Chemical Name】Selexipag
【Original】Actelion
【Time to market】2015.12.21
【Patents expire】2023.04.04
【Use】Oral Selexipag tablets are used to treat pulmonary arterial hypertension (PAH).
Selexipag
一、 Product Overview
Selexipag, used to treat pulmonary arterial hypertension (PAH), was originally developed by Nippon neopharmaceutics and later licensed to actel for co-development. On December 21, 2015, it was approved by the food and drug administration (FDA) and the patent of the compound expired on April 4, 2023.
二、Main products
Description | Structural Formula | CAS No. | Category |
Selexipag | 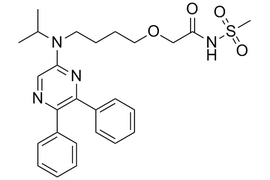 | 475086-01-2 | API |
4-(Isopropylamino)butanol | 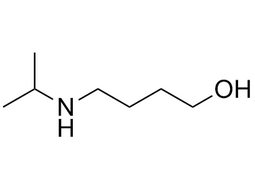 | 42042-71-7 | intermediates |
4-[(5,6-diphenyl-2-pyrazinyl)(1-methylethyl)amino]-1-Butanol | 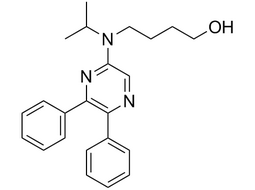 | 475086-75-0 | intermediates |
5-chloro-2,3-diphenylpyrazine | 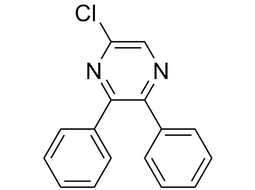 | 41270-66-0 | intermediates |
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.


