Suvorexant
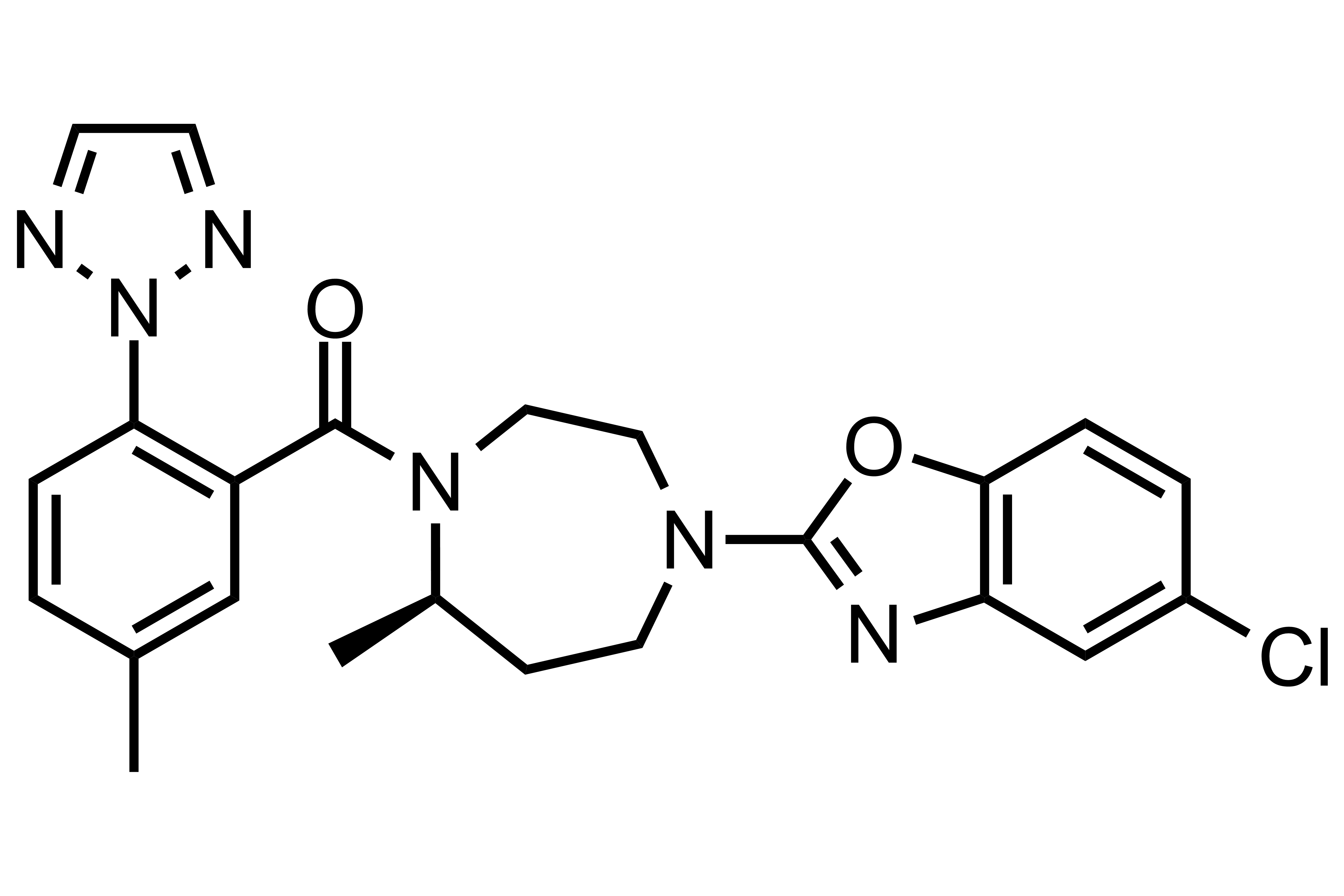
Suvorexant
【Chemical Name】Suvorexant
【Original】MSD
【Time to market】2014.8.13
【Patents expire】2027.11.30
【Dosage and Usage】Tablets, 5 mg, 10 mg, 15 mg, 20 mg. used for the treatment of insomnia, characterized by difficulties with sleep onset and/or sleep maintenance.
Suvorexant
一、Product Overview
Suvorexant, developed by Merk Sharp & Dohme (MSD) ,used for the treatment of insomnia, characterized by difficulties with sleep onset and/or sleep maintenance. It was approved by the FDA on August 13, 2014 and approved by the PMDA on September 26, 2014. The compound patent will expire on November 30, 2027.
二、Product Overview
Description | Structural Formula | CAS No. | Category |
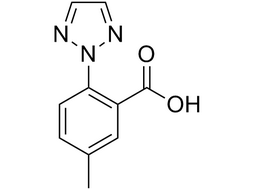
5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoic acid |
| 956317-36-5 | intermediates |
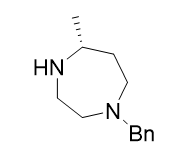
(R)-1-benzyl-5-methyl-1,4-diazepane |
| 1620097-06-4 | intermediates |
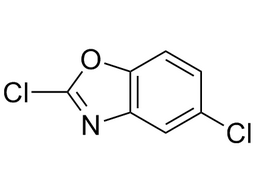
2,5-Dichlorobenzooxazole |
| 3621-81-6 | intermediates |
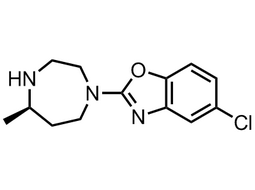
(R)-5-chloro-2-(5-methyl-1,4-diazepan-1-yl)benzo[d]oxazole |
| 1266975-27-2 | intermediates |
※ Only provide intermediates.
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.