Lumateperone Tosylate
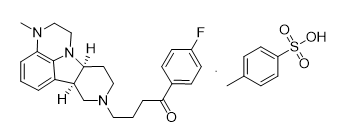
Lumateperone Tosylate
【Chemical Name】Lumateperone Tosylate
【Original】Bristol-Myers Squibb
【Time to market】2019.12.20
【Dosage and Usage】Capsules: 42 mg, 21 mg, 10.5 mg .used for the treatment of Schizophrenia in adults and depressive episodes associated with bipolar I or II disorder (bipolar depression) in adults, as monotherapy and as adjunctive therapy with lithium or valproate
Lumateperone Tosylate
一、 Product Overview
Lumateperone Tosylate is developed by Bristol-Myers Squibb which is an atypical antipsychotic indicated for the treatment of Schizophrenia in adults and depressive episodes associated with bipolar I or II disorder (bipolar depression) in adults, as monotherapy and as adjunctive therapy with lithium or valproate.It was approved by the US Food and Drug Administration (FDA) on Dec.20, 2019
二、Main products
Description | Structural Formula | CAS No. | Category |
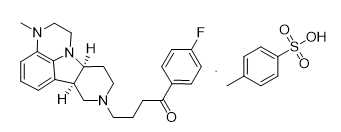
Lumateperone Tosylate |
| 1187020-80-9 | API |
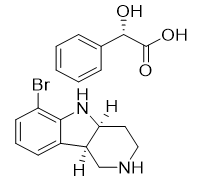
4aS,9bR)-6-Bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole (S)-2-Hydroxy-2-phenylacetate |
| 1059630-13-5 | intermediates |
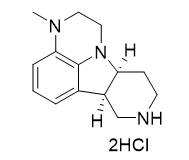
(6bR,10aS)-3-methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline dihydrochloride |
| 2339905-28-9 | intermediates |
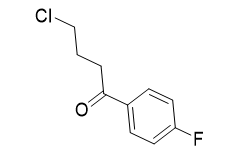
4-chloro-1-(4-fluorophenyl)butan-1-one |
| 41167-07-1 or 3874-54-2 | intermediates |
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.