Gadoxetate Disodium
Gadoxetate Disodium
【Chemical Name】 Gadoxetate Disodium
【Original】Bayer
【Time to market】2004.3.26
【Dosage and Usage】Injection 1.8143GM/10ML (181.43MG/ML);2.72145GM/15ML (181.43MG/ML),
indicated for intravenous use in T1-weighted magnetic resonance imaging (MRI)
of the liver to detect and characterize lesions in adults with known or suspected focal liver disease.
Gadoxetate Disodium
一、 Product Overview
Gadoxetate Disodium is a gadolinium-based contrast agent indicated for intravenous use in T1-weighted magnetic resonance imaging (MRI) of the liver to detect and characterize lesions in adults with known or suspected focal liver disease. On March 26, 2004, it was approved by Sweden.On October 19, 2007, it was approved by PMDA and on July 3, 2008, it was approved by FDA.
二、Main products
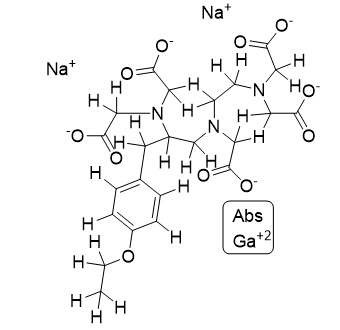
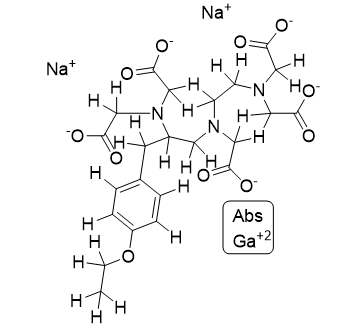
Description | Structural Formula | CAS No. | Category |
Gadoxetate Disodium |
| 135326-22-6 | API |
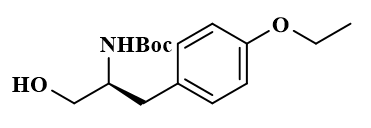
Tert-butyl (S)-(1-(4-ethoxyphenyl)-3-hydroxypropan-2-yl)carbamate |
| 1252686-29-5 | intermediates |
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.