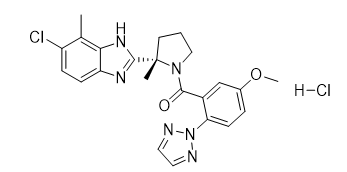
Daridorexant Hydrochloride
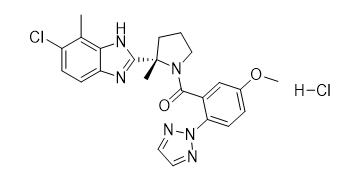
Daridorexant Hydrochloride
【Chemical Name】Daridorexant Hydrochloride
【Original】Actelion
【Time to market】2022.01.07
【Dosage and Usage】Tablets: 25 mg, 50 mg,used for the treatment of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenance.
Daridorexant Hydrochloride
一、 Product Overview
Daridorexant Hydrochloride is a small molecule drug developed by Actelion Pharmaceuticals Ltd and approved by the US Food and Drug Administration (FDA) on 07 January 2022 for the treatment of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenance.
二、Main products
Description | Structural Formula | CAS No. | Category |
Daridorexant Hydrochloride |
| 1792993-84-0 | API |
4-Chloro-3-Methylbenzene-1,2-diaMine hydrochloride |
| 1087743-89-2 | intermediates |
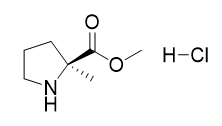
2-Methyl-L-proline Methyl ester hydrochloride |
| 220060-08-2 | intermediates |
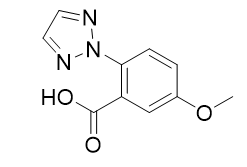
5-methoxy-2-(2H-1,2,3-triazol-2-yl)benzoic acid |
| 1293284-55-5 | intermediates |
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.