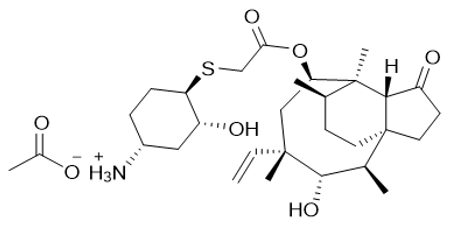
Lefamulin Acetate
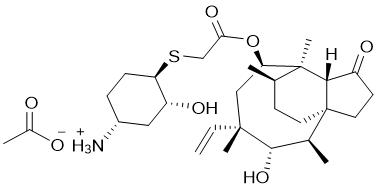
Lefamulin Acetate
【Chemical Name】Lefamulin Acetate
【Original】Nabriva Therapeutics
【Time to market】2021.01.28
【Dosage and Usage】Injection : A single-dose clear glass vial containing 150 mg of
lefamulin in 15 mL of 0.9% sodium chloride for further dilution prior to intravenous
infusion.
Tablets: 600 mg of lefamulin. Used for the treatment of adults with community-acquired
bacterial pneumonia (CABP) caused by susceptible microorganisms.
Lefamulin Acetate
一、 Product Overview
Lefamulin Acetate,developed by Nabriva Therapeutics, is a pleuromutilin antibacterial indicated for the treatment of adults with community-acquired bacterial pneumonia (CABP) caused by susceptible microorganisms.It was approved by FDA on Aug.19, 2019
二、Main products
Description | Structural Formula | CAS No. | Category |
Lefamulin acetate |
| 1350636-82-6 | API |
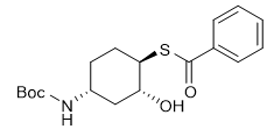
S-((1R,2R,4R)-4-((tert-butoxycarbonyl)amino)-2-hydroxycyclohexyl) benzothioate |
| 1350636-89-3 | intermediates |
S-((1R,2R,4R)-2-hydroxy-4-(2,2,2-trifluoroacetamido)cyclohexyl) benzothioate | 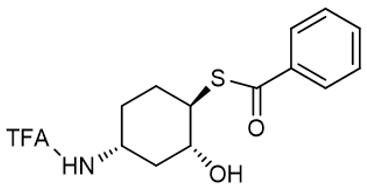 | 1350636-92-8 | intermediates |
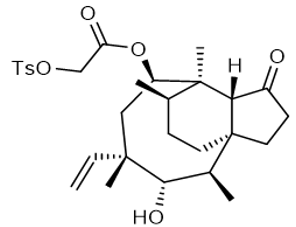
TOS- pleu |
| 31716-01-5 | intermediates |
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.