Palonosetron Hydrochloride
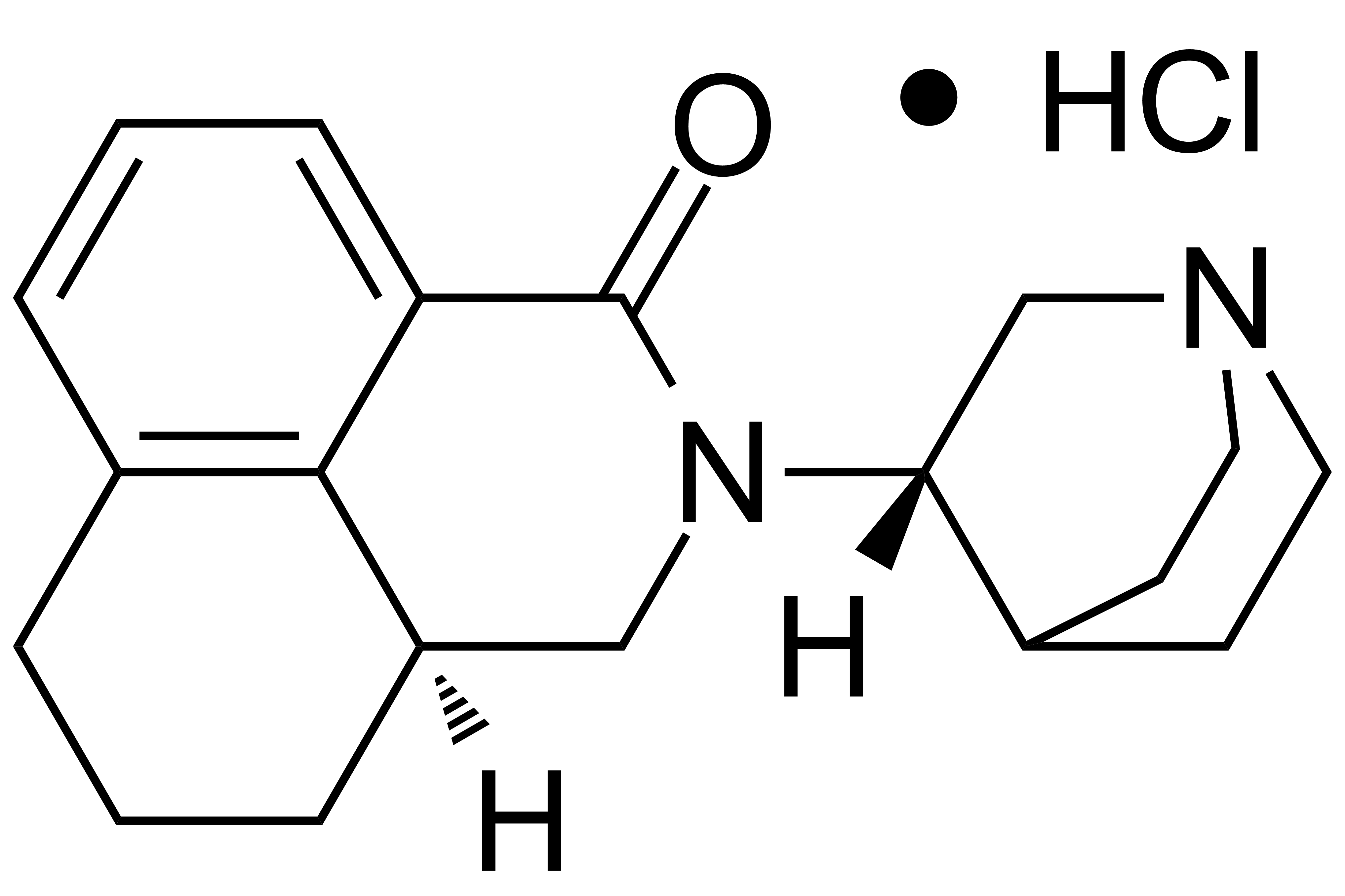
Palonosetron HCl
【Chemical Name】Palonosetron Hydrochloride
【Original】Helsinn Healthcare
【Time to market】2003.7.25
【Patents expire】2024.1.30
【Dosage and Usage】Injection, containing 0.25 mg/5 mL or 0.075 mg/1.5 mL,
used for the treatment of acute and delayed nausea and vomiting caused by
chemotherapy for primary and repeated courses of moderately.
Palonosetron HCl
一、 Product Overview
Palonosetron HCl by Helsinn Healthcare development, used to prevent the first time and repeat treatment of moderate or high acute vomiting caused by cancer chemotherapy and the late-occurred nausea and vomiting, and prevention of postoperative nausea and vomiting (PONV) within 24 hours, on July 25, 2003, the United States food and drug administration (FDA) approved compound patents expire on January 30, 2024.
二、Main products
Description | Structural Formula | CAS No. | Category |
Palonosetron Hydrochloride | 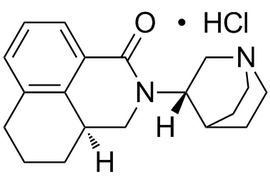 | 135729-62-3 | API |
5,6,7,8-Tetrahydro-1-naphthoic acid | 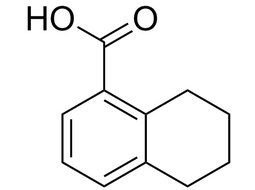 | 4242-18-6 | Intermediate |
(S)-(-)-1,2,3,4-Tetrahydro-1-naphthoic acid | 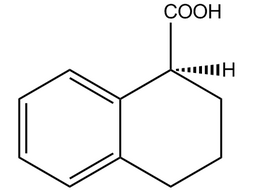 | 85977-52-2 | Intermediate |
(S)-(-)-3-aminoquinuclidine dihydrochloride |  | 119904-90-4 | Intermediate |
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.



