Brivaracetam
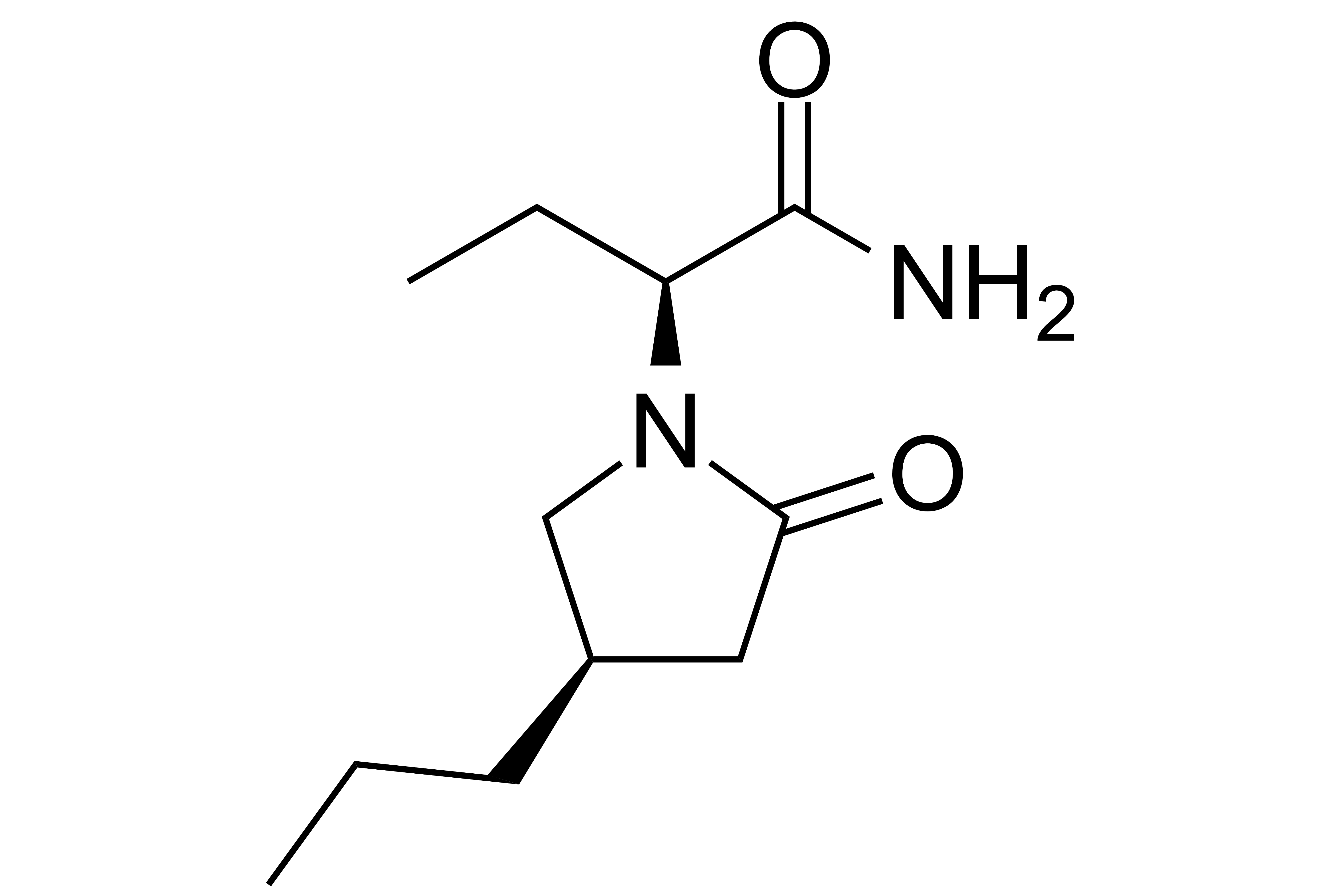
Brivaracetam
【Chemical Name】Brivaracetam
【Original】UCB
【Time to market】2016.02.18
【Patents expire】2021.02.21
【Dosage and Usage】Tablets:10 mg, 25 mg, 50 mg, 75 mg, and 100 mg,Oral solution:10 mg/mL,
Injection: 50mg/5 mL single-dose vial,used for the treatment of partial- onset seizures in patients 1 month of age and older
Brivaracetam
一、 Product Overview
brivaracetam by optimal ratio (UCB) company research and development, for the treatment of adults and teenagers aged 16 partial seizure epilepsy patients, with or without secondary systemic adjuvant therapy of the attack in January 14, 2016, the European drug administration approval (EMA), then in February 18, the same year the FDA approal, compound patent feb 21, 2021 expiration date.
二、Main products
Description | Structural Formula | CAS No. | Category |
Brivaracetam | 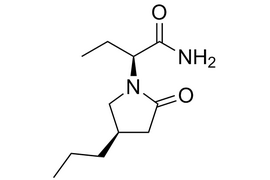 | 357336-20-0 | API |
5-hydroxy-4-propylfuran-2(5H)-one | 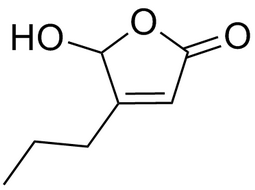 | 78920-10-2 | intermediates |
L-2-Aminobutanamide hydrochloride | 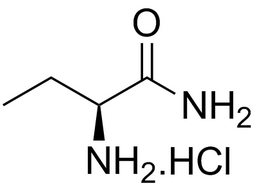 | 7682-20-4 | intermediates |
(R)-4-propyl-dihydro-furan-2-one | 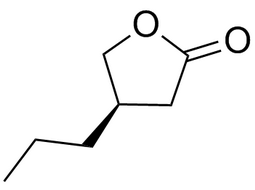 | 63095-51-2 | intermediates |
Note: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement and its liability is at buyer's risk.



